President Trump's COVID-19 Treatment Includes These Drugs
Trump received an infusion of antibodies on Friday, the White House said.
President Trump's COVID-19 Treatment Includes These Drugs
The list of treatments President Donald Trump has received for his coronavirus infection range from experimental to over the counter. Here are the four most notable.
Antibody therapy
Trump received an infusion of antibodies on Friday, the White House said. These germ-blocking proteins aim to prevent the coronavirus from entering cells and causing infection. Our immune systems normally make antibodies on their own, but it can take weeks for them to appear in response to a new infection such as the coronavirus. Injecting lab-grown antibodies offers a shortcut.
Drugmaker Regeneron produced the dual-antibody cocktail Trump received. The therapy is experimental and has not been approved by the U.S. Food and Drug Administration. Trump received it through the company's "compassionate use" program, which Regeneron said is "intended for patients with serious or life-threatening conditions who do not have any viable or available treatment options."
The product is one of two antibody therapies undergoing late-stage clinical trials. Both Regeneron and the other product's manufacturer, Eli Lilly, recently announced encouraging results in press releases, but their data have not been reviewed by other experts.
"It's, I think, a promising therapy. It's not proven," said Rajesh Gandhi, an infectious diseases physician at Massachusetts General Hospital and Harvard Medical School. Gandhi helped write COVID-19 treatment guidelines for the National Institutes of Health and the Infectious Diseases Society of America.
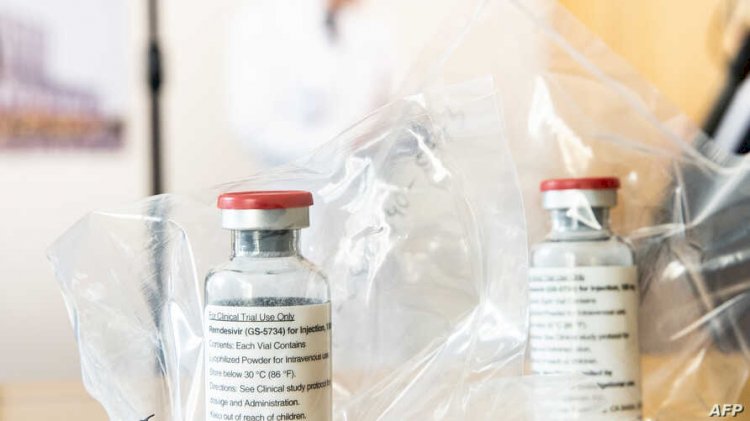
Remdesivir
While antibody therapy is being tested for patients with mild disease, Trump received an antiviral drug on Saturday that is normally given to patients in worsening condition and which was originally developed to treat another viral disease, Ebola.
Trump's oxygen levels dropped below a critical threshold sometime Saturday.
"That's where the antiviral remdesivir has its greatest role," Gandhi said.
Manufactured by drugmaker Gilead, remdesivir is the first new anti-coronavirus drug to receive emergency use authorization from the FDA.
The drug works by interfering with the machinery the virus uses to make copies of its genetic instructions.
It is not a panacea, however. It has not been shown to lower death rates, for example. It shortened hospital stays in one study. Another study found patients receiving remdesivir fared better than those getting a placebo, but not by much.
Supplies of the drug have improved since the spring, Gandhi said, and the company is ramping up production.
Dexamethasone
The fact that Trump was given dexamethasone suggests that at least at some point his condition was serious.
"In mild disease, we don't give dexamethasone because it doesn't help people who aren't on oxygen and it may actually be harmful," Gandhi said.
The cheap, widely available steroid does not work on the virus itself, but instead treats the side effects caused by the body's response to the virus.
"That inflammatory response is trying to battle off the virus," Gandhi said. "but sometimes it can itself lead to low lung function."
The drug saved lives in a study. It lowered the death rate by about a third in patients on ventilators and by 18% in patients on supplemental oxygen. It didn't help people who were less sick, however.
Famotidine
Trump also took the over-the-counter antacid famotidine, which goes by the brand name Pepcid, but the reason is not clear.
It's not uncommon for a patient being hospitalized to receive an antacid, Gandhi said.
"It’s a stressful time, and people are having heartburn or other symptoms," he noted.
On the other hand, "a few months ago, people had wondered about whether famotidine might have a role for treating COVID-19," Gandhi added. "But the evidence for that is still lacking."
Trump in the past has supported unproven treatments, including the anti-malaria drug hydroxychloroquine. A U.S. National Institutes of Health study ended early because it found no benefit from taking the drug.
Famotidine is "not a harmful drug. I could walk out and get it from my pharmacy," Gandhi said. "But we just don't know if it works."
VOA






































